Efficacy and safety of secukinumab in Chinese patients with moderate-to-severe plaque psoriasis: a real-life cohort study | Chinese Medical Journal

Estimated relationships for PaSI90, PaSI100, and sPGa0/1 responses at... | Download Scientific Diagram
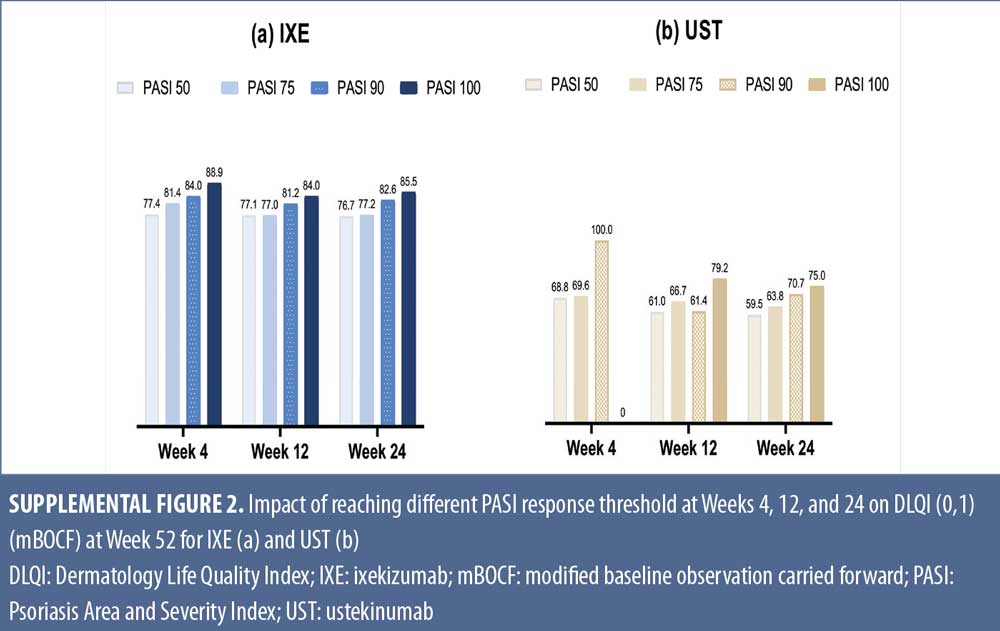
Early Treatment Targets for Predicting Long-term Dermatology Life Quality Index Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post-hoc Analysis from a Long-term Clinical Study – JCAD | The Journal of Clinical
Real-world efficacy of biological agents in moderate-to-severe plaque psoriasis: An analysis of 75 patients in Taiwan | PLOS ONE

Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis | Chinese Medical Journal

Guselkumab Was More Effective Than Secukinumab in Patients with Plaque Psoriasis and the Subset of Patients with Self-Reported Psoriatic Arthritis in a Randomized, Double-blind, Head-to-head Comparison Study over 1 Year - ACR

Clinical outcomes at 1 year in early Psoriasis Area and Severity Index responders compared with non‐responders: Subgroup analysis of UNCOVER‐3 trial - Rosmarin - 2021 - Skin Health and Disease - Wiley Online Library

Absolute Versus Relative Psoriasis Area and Severity Index in Clinical Practice | Actas Dermo-Sifiliográficas

Percentage of PASI 75, PASI 90, PASI 100 and PGA responders at weeks 12... | Download Scientific Diagram

Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52‐week, open‐label study - Yamasaki - 2017 - British Journal of Dermatology - Wiley Online Library
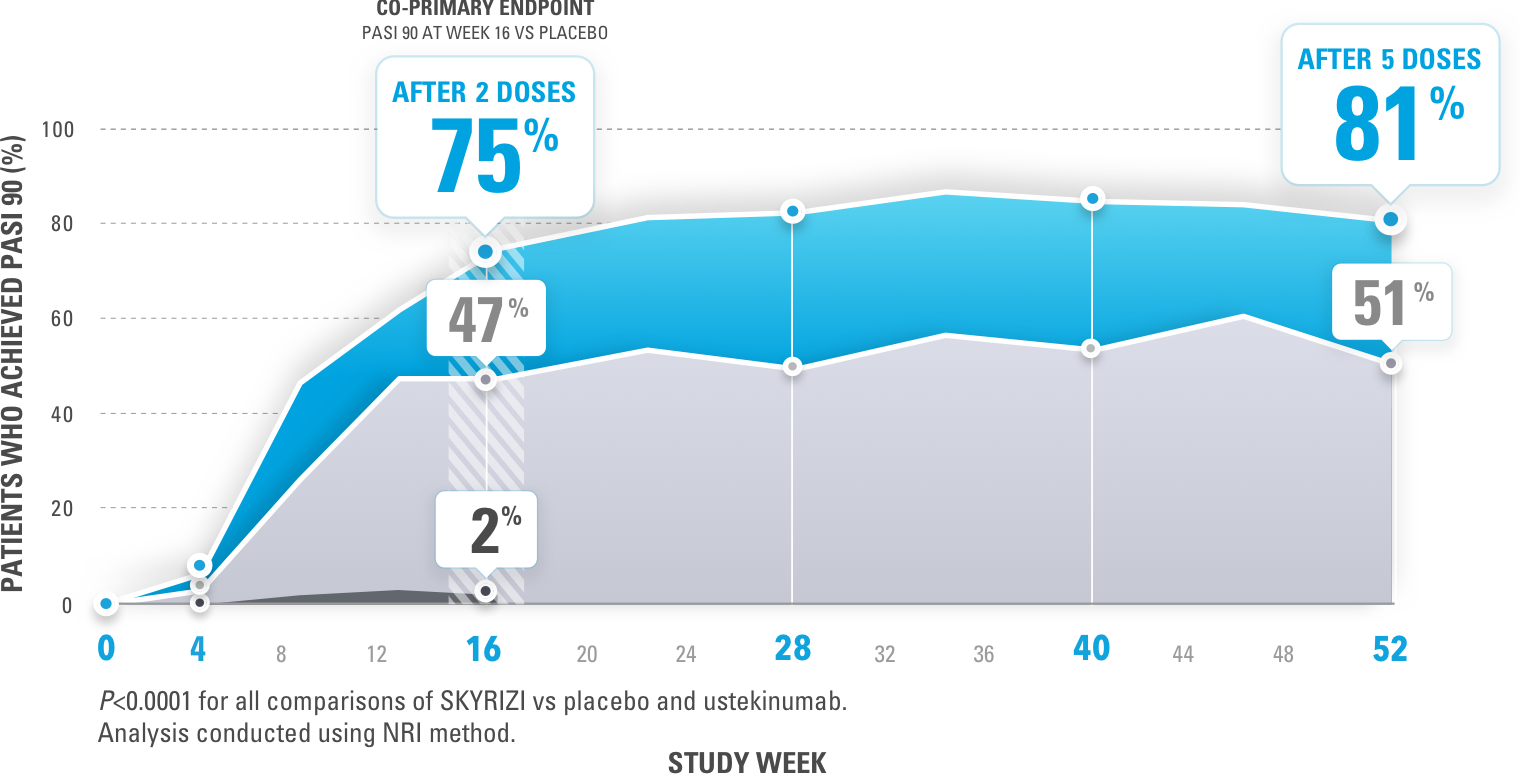
New Novartis data shows Cosentyx™ is significantly superior to Stelara® and clears skin (PASI 90) in nearly 80% of psoriasis patients | Novartis

Improvement in Psoriasis Symptoms and Physical Functioning with Secukinumab Compared with Placebo and Etanercept in Subjects with Moderate-to-Severe Plaque Psoriasis and Psoriatic Arthritis: Results of a Subanalysis from the Phase 3 Fixture

Clinical response after guselkumab treatment among adalimumab PASI 90 nonresponders: Results from the VOYAGE 1 and 2 trials | Semantic Scholar